A wide range of news on Hana Pharm
Efficacy and Safety are confirmed.
Expectations rise as the new anesthetic drug is developed after 20 years.
Hana Pharm (293480, Representative Director Yun-ha Lee), a pharmaceutical company specialized in anesthetic and analgesic drugs, announced on June 11 that the latest results of clinical trials of ‘Remimazolam’, which receives an attention as the new anesthetic drug, were presented at the International Congress of Cardiothoracic and Vascular Anesthesia (ICCVA-ASCA 2019) that was recently held in COEX in Seoul, Korea.
For the conference, Professor Jae-hyeon Park of Anesthesiology and Pain Medicine in Seoul National University College of Medicine was participated as the senior person present and Professor Joerg Fechner of Department of Anesthesiology in Friedrich-Alexander University Erlangen-Nuremberg gave a presentation on ‘New Challenges and Approaches for Total Intravenous Anesthesia in Cardiothoracic Surgery’.
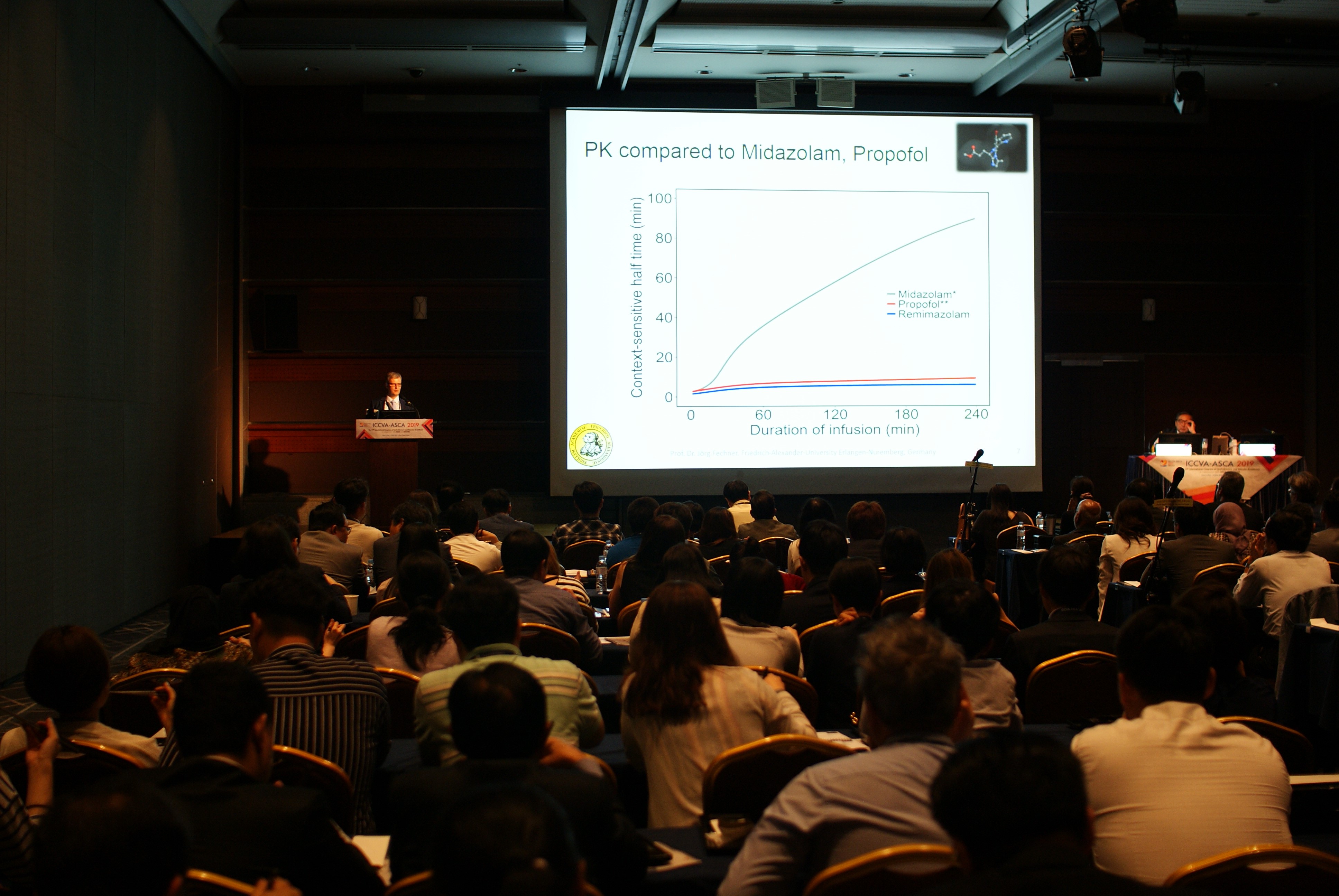
“Based on the results of large scale clinical trials conducted in Korea and Europe, Remimazolam showed equivalent efficacy compared to that of Propofol and its excellence was proved as the amount of norepinephrine used in occurrence of hemodynamic side effects was less than that used in Propofol,” said Professor Joerg Fechner. “Particularly, an occurrence of side effects such as decrease in blood pressure, hypotension, and decrease in heart rate and bradycardia in Remimazolam was lower than that in Propofol, verifying its safety. Remimazolam can be reversed with flumazenil and it is excellent for anterograde amnesia after anesthesia compared to Propofol,” he added and emphasized once more.
Prior to this, Hana Pharm conducted Phase III clinical trials on 198 patients who were planned to undergo general anesthesia from March to October last year and the final report was presented in last February. Hana Pharm signed an exclusive contract with Paion of Germany for research and development, manufacturing, distribution and control of Remimazolam in Korea.
One Hana Pharm official said, “There was a high expectation for launch of Remimazolam during the conference where professionals of anesthesiology and pain medicine from all over the world were participated and in Korea, where there was a social issue such as Propofol abuse, Propofol is currently classified as an anesthetic drug and is strictly being controlled. As Remimazolam is the new global anesthetic drug after 40 years, it would replace those of the previous drugs in the market.”
(End)