The Hana Pharm R&D division established in 1997 to implement the company’s corporate philosophy of “Best medicine! Better life!” and is producing high-value-added specialty medicines through research in synthesis and formulation of drugs.
Also we always try to develop best medicine with talented researchers superior research facilities, and high technology. In the future, Hana Pharm will continue to do the best to lead the world market by developing new drugs with our corporate philosophy, “We are committed to better life with the best medicine.”
-
-
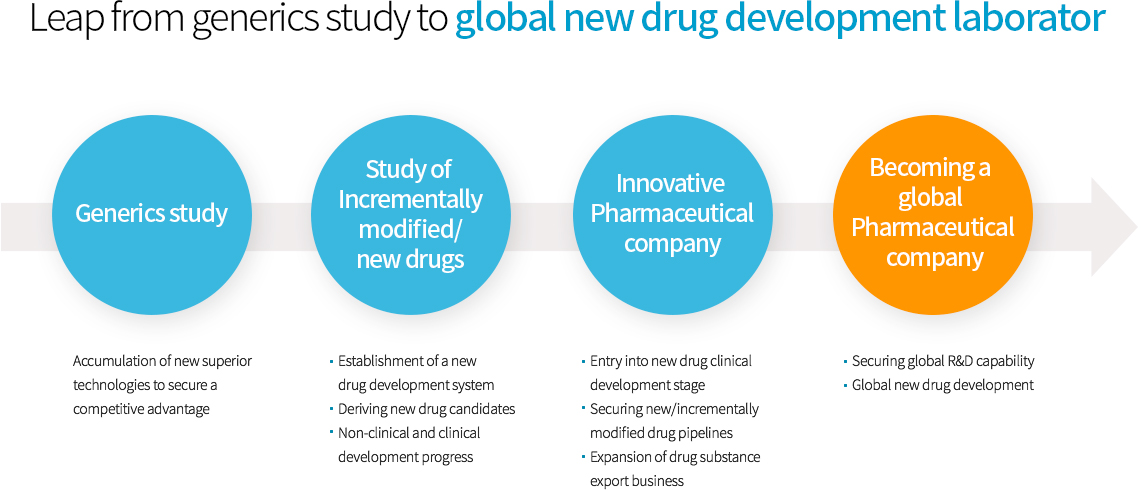
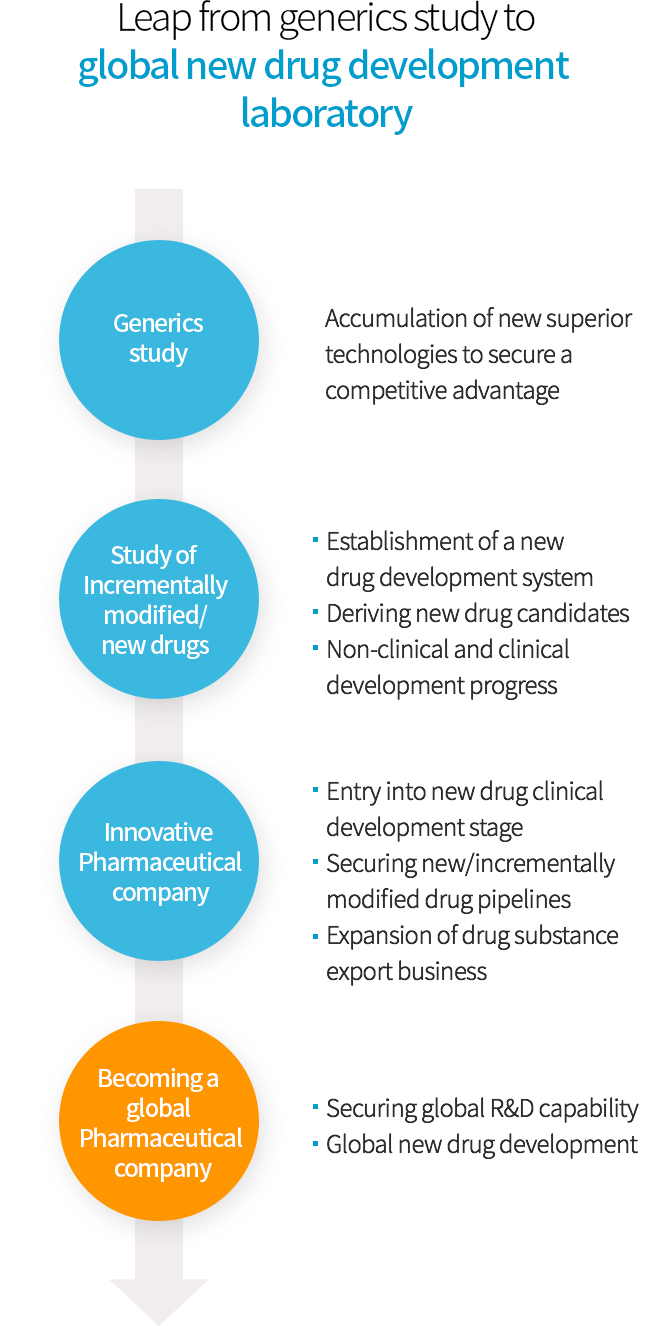
Leap from generics study to global new drug development laboratory
- Generics study
-
- Accumulation of new superior technologies to secure a competitive advantage
- Study of Incrementally modified/ new drugs
-
- ㆍEstablishment of a new drug development system
- ㆍDeriving new drug candidates
- ㆍNon-clinical and clinical development progress
- Innovative Pharmaceutical company
-
- ㆍEntry into new drug clinical development stage
- ㆍSecuring new/incrementally modified drug pipelines
- ㆍExpansion of drug substance export business
- Becoming a global Pharmaceutical company
-
- ㆍSecuring global R&D capability
- ㆍGlobal new drug development
Major Business Objectives
-
Global expansion
Strengthening synthetic drug business and begin overseas export
-
Leading generic research
Market needs analysis, process systematization/quality improvement, timely approval and release
-
Growing as a new drug development laboratory
Strengthening global network and securing pipeline through open innovation
-
-
2018
- Selection and implementation of governmental tasks on the development of novel drug having VEGF(R) inhibitory properties for diabetic retinopathy. (Ministry of Health & Welfare)
- Selection and implementation of governmental tasks on biolipid's interactomics research center. (Ministry of Science and ICT)
-
2017
- Establishment of research planning office
- BA/BE approval of Polaprezinc, Cuparin Tab., and Codeine Phosphate Tab. Hana
- Selection and implementation of governmental tasks on the development of a safety-improved cyclic gadolinium magnetic resonance imaging T1 contrast media (Ministry of Industry and Commerce)
- MF registration for Granisetron drug substance export to Japan
-
2016
- Opening of Pangyo R&D Center and
establishment of bio-pharmacological lab
- BA/BE approval of Covaratan Tab., Atorvastatin calcium,
Risedronate sodium, Tasline Tab.,and MSR SR Tab
- Development of safe and effective nanotechnology-based drug delivery system (DDS) (MFDS), Selection and implementation of governmental tasks on the development of natural health functional foods and cosmetics materials (Ministry of SMEs and Startups) using Moringa
-
2015
- BA/BE approval of Telmidipine Tab, Tramiphen Tab., Tramiphen Semi Tab., and Eltale.
-
2014
- BA/BE approval of Telmisartan, Amlodipine/Valsartan, Rosuvastatin, Oxycodone HCL, Varidase Tab, and Arito Tab.
-
2013
- BA/BE approval of Almati Tab., Busone Tab., Tomax Tab., Ibesa Tab.,and Rosto Tab.
-
2011
- Technology transfer agreement with Kyungpook National University for MRI Macrocyclic contrast media compound
-
2010
- Completion of Hagil Central Laboratory
-
2006
- Reorganization of the laboratory (synthesis laboratory, process laboratory, formulation laboratory, analytical laboratory)
-
2003
- Expansion of the Central Laboratory
-
1999
- Completion of the Central Laboratory (attached synthesis room)
-
1998
- Establishment of the Sangshin Central Laboratory
- Selection as a military service exemption laboratory