Byfavo
A Novel Ultra-Short Acting Anesthetic/Sedative Under Development at Hana Pharm

A Novel Ultra-Short Acting Intravenous GABA Alpha Receptor Modulator
I. Soft Byfavo
Soft drug design represents a new approach aimed to design safer drugs with an increased therapeutic index by integrating metabolism considerations into the drug design process. They are therapeutic agents that undergo predictable metabolism to convert into inactive metabolites after exerting their therapeutic effect. They are designed by building into the molecule, in addition to the activity, the most desired way in which the molecule is to be deactivated and detoxified.1]II. Fast Byfavo
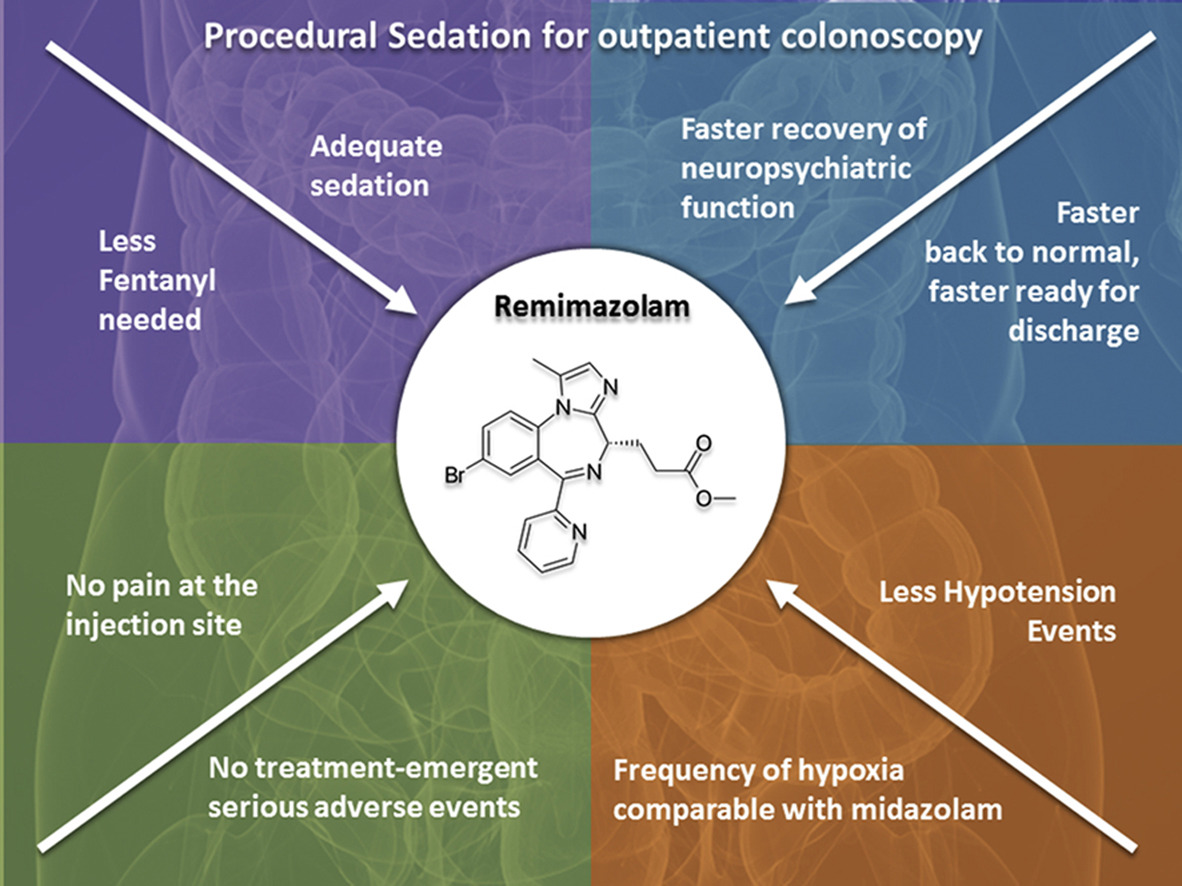
Aspects of soft drugs designs that are particularly interesting to anesthesiologists include rapid onset of effect and quick recovery. 3]III. Clean Byfavo
Byfavo shows high extrahepatic clearance, and since its metabolism does not rely on cytochrome enzymes, metabolic interactions with other drugs in the liver is limited. 7] A Phase I trial confirmed its safety even in Japanese patients with mild to moderate hepatic disorders. 11] Also in general anesthesia, no dose adjustment was necessary even in patients with end-stage renal disease. 11]Unparalleled Novelty of Byfavo "Independency"
1. Organ-independent elimination: Byfavo can be administered safely in patients with hepatic or renal impairment. 2,11]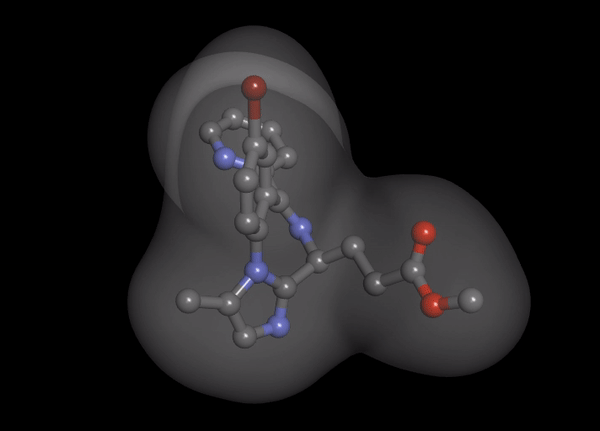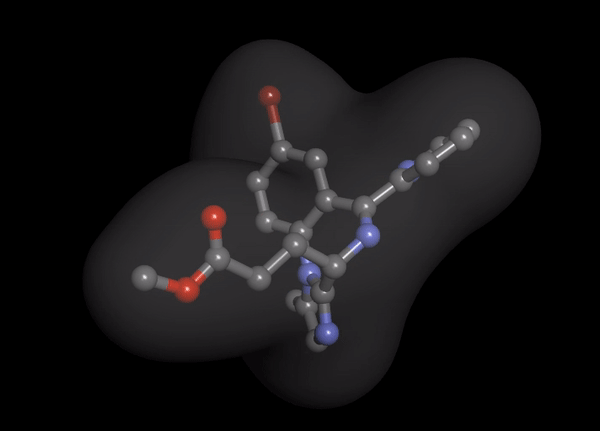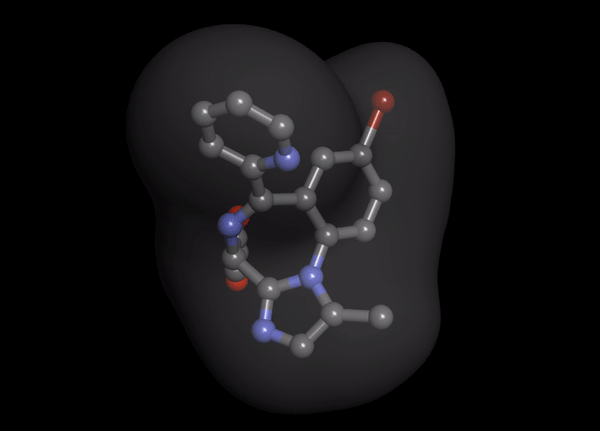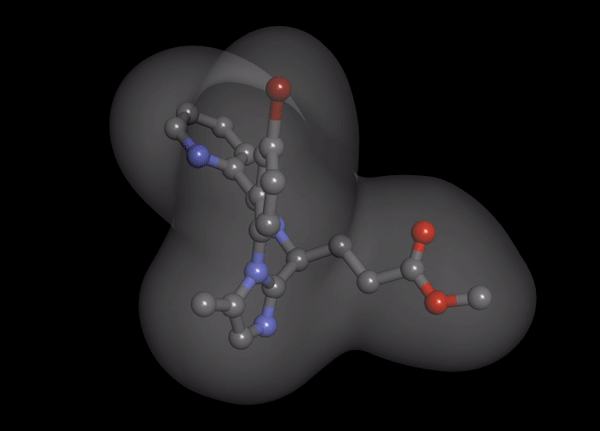

PAION
EU

ACACIA PHARMA
US

MUNDI PHARMA
Japan

YICHANG HUMANWELL
China

PHARMASCIENCE
Canada

R-PHARM
Russia
Last updated --
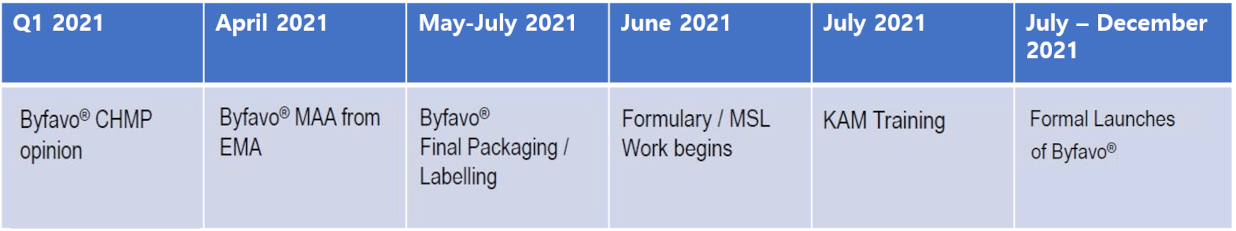
Last updated --
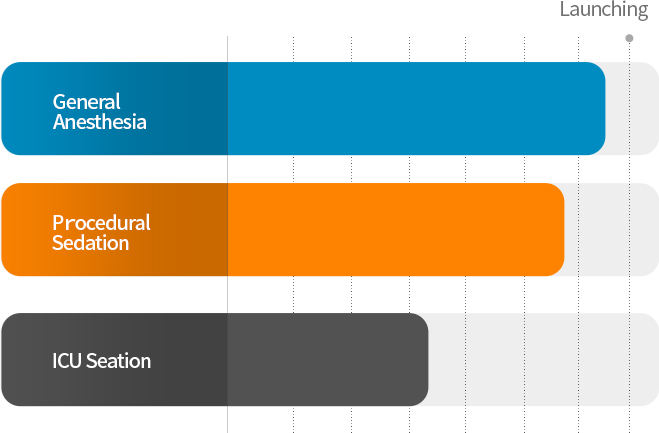
▶ Approved in General Anesthesia in January 2021